Dmitri Mendeleev was a Russian chemist who is famous as the Father of the Periodic Table. He formulated the Periodic law and popularized the periodic table through his correct predictions regarding the properties of yet undiscovered elements. Mendeleev had a tough childhood during which his family faced financial difficulties. After his graduation, he worked as a professor at the Saint Petersburg University and wrote many path-breaking books and papers on chemistry. There are numerous legends and controversies associated with Mendeleev. His second marriage caused an uproar in Russia and he was denied a Nobel Prize due to politics in the Academy. There is a legend that he saw the arrangement of his periodic table in a dream, which might be true; and another legend says that he set the strength of Vodka to 40%, which is definitely untrue. Here are 10 interesting facts about the childhood, education, life, family, career and death of Dmitri Mendeleev.
#1 HIS CHILDHOOD WAS MARRED BY TRAGEDIES IN THE FAMILY
Dmitri Ivanovich Mendeleev was born on February 8, 1834 in the village of Verkhnie Aremzyani, near Tobolsk, in the Russian province of Siberia. Dmitri was part of a large family and he had more than 10 siblings, the exact number is not known. His father Ivan Pavlovich Mendeleev was a teacher of fine arts, politics and philosophy. Unfortunately for the family, Ivan went blind and lost his teaching position. His mother Maria Dmitrievna Mendeleeva restarted her family’s abandoned glass factory to support the family. Dmitri’s father died when he was only 13 and two years later his mother’s glass factory was destroyed in a fire leading to a financial crisis in the family.

#2 HE GRADUATED WITH A GOLD MEDAL FROM THE MAIN PEDAGOGICAL INSTITUTE
Dmitri Mendeleev initially attended the Gymnasium, or a school with strong emphasis on academic learning, in Tobolsk. In 1850, at the age of 16, the Mendeleev family moved to the Russian capital Saint Petersburg. Here, he entered the Main Pedagogical Institute, a teacher training institute from where his father had also graduated. Dmitri graduated with a gold medal in 1855. After briefly occupying a teaching position at Simferopol in Crimea, Dmitri returned to St. Petersburg and earned a Master’s degree from the University of St. Petersburg for his study of silicates, in 1856, at the age of 22. He won an award to go to Western Europe to pursue chemical research. He stayed in Europe for two years from 1859 to 1861, spending most of his time at the University of Heidelberg in Germany.

#3 MENDELEEV PLAYED A MAJOR ROLE IN TRANSFORMING CHEMISTRY IN RUSSIA
After his time in Europe, Mendeleev believed that Russia was trailing behind in the science of chemistry. In 1861, he published his textbook Organic Chemistry which won him the prestigious Demidov Prize and put him at the forefront of Russian chemical education. Mendeleev became a professor at Saint Petersburg Technological Institute and Saint Petersburg State University in 1864 and 1865 respectively. He was one of the founders, in 1869, of the Russian Chemical Society. His renowned work The Principles of Chemistry, was published in two volumes (1868–71). It became the definitive textbook on the subject and was widely translated. By 1871, Mendeleev had transformed St. Petersburg into an internationally recognized center for research in chemistry.

#4 HE MIGHT HAVE ENVISIONED THE PATTERN OF THE PERIODIC TABLE IN A DREAM
In 1869, while preparing the second volume of The Principles of Chemistry, Mendeleev attempted to classify the elements according to their chemical properties and noticed patterns. He wrote the name of all the known elements with their fundamental properties, including atomic weight, on cards. He noticed that the behavior of elements seemed to repeat as their atomic weights increased. The legend goes that, while trying to figure out a pattern he fell asleep at his desk and when he awoke, he knew the complete arrangement of the elements. It is proposed that his subconscious mind did the trick for him. Mendeleev did claim the dream story according to certain sources but other sources regard it as untrue. On 6 March 1869, Dmitri Mendeleev made a presentation to the Russian Chemical Society in which he stated his periodic law, which states that when elements are ordered according to their atomic weights, certain properties of elements repeat periodically.
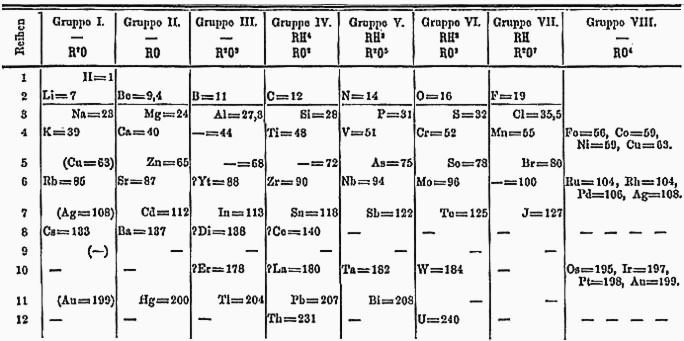
#5 He CORRECTLY PREDICTED THE EXISTENCE, AND PROPERTIES, OF YET UNDISCOVERED ELEMENTS
Mendeleev was not the first to arrange the elements according to their atomic weights, neither was he the first to notice periodicity of elements. However, he was the first to publish a periodic table that closely resembles the modern one and more importantly, it was his efforts that popularized the Periodic Table. Mendeleev made two spectacular predictions. Firstly, he put the elements in their correct places despite some atomic weights being incorrect at the time and rightly predicted that these atomic weights had been measured incorrectly. Secondly, he left gaps in his periodic table for elements which were not yet discovered and predicted the properties of these elements. The Periodic Table gained acceptance in the scientific community in the following two decades with three elements predicted by Mendeleev being discovered with properties closely matching what he had predicted. It is due to these contributions that Dmitri Mendeleev is considered the Father of the Periodic Table.
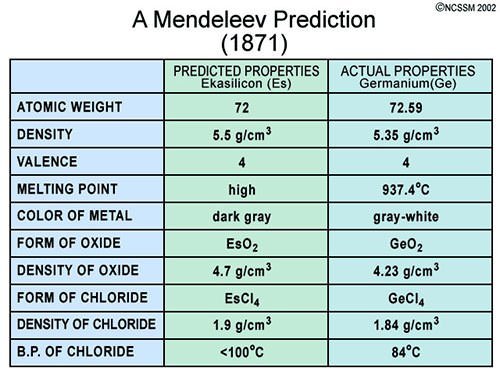
#6 HE USED WORDS OF SANSKRIT AS PREFIXES OF MISSING ELEMENTS IN HIS TABLE
Mendeleev was an admirer of ancient Indian scholar Pāṇini, who found that the phonological patterning of sounds in a language is a function of their articulatory properties. Panini is considered by some as the father of linguistics. Mendeleev named the missing elements in his Periodic Table with the prefixes of eka, dvi and tri (Sanskrit one, two, three) to honor Sanskrit grammarians, like Panini, of ancient India. Like, he referred to scandium and gallium as eka-boron and eka-aluminum. Apart from the Periodic Table, other achievements of Mendeleev include important contributions in determining the nature of solutions, introducing the metric system in Russia, defining the critical temperature of a gas and inventing a smokeless powder named pyrocollodion.

#7 HIS SECOND MARRIAGE WAS UNLAWFUL AND CREATED A HUGE UPROAR IN RUSSIA
In 1862, Dmitri Mendeleev, on suggestion of his sister Olga, married Feozva Nikitichna Leshcheva. The couple had two children, a boy named Volodya and a daughter named Olga. However, in 1876, 43 year old Mendeleev fell hopelessly in love with the 19 year old Anna Ivanova Popova, his niece’s best friend. He began courting her and in 1881, he proposed to her, threatening to commit suicide if she refused. Mendeleev and Anna Popova married on April 2 the following year, one month before Mendeleev’s divorce with Leshcheva was finalized. Russian Orthodox Church required at least seven years before lawful remarriage but Mendeleev violated the prohibition to a great deal of public uproar. His decision contributed to his failure in being elected into Russia’s Academy of Science at the time. Despite their difference of age, Mendeleev’s marriage to Popova is considered successful. The couple had four children, Liubov, Ivan, and twins Vassili and Maria.

#8 He NEVER SET THE 40% STANDARD STRENGTH OF VODKA DESPITE THE POPULAR BELIEF
Mendeleev had a great interest in shipbuilding and wrote over 40 scientific papers on the subject. He helped create Russia’s first ship model basin for testing of ship designs and he took part in designing Yermak, the first polar icebreaker in the world. Mendeleev was also an enthusiastic traveler and photographer. He was fond of crafting his own bags and suitcases; and there is a legend that merchants in the market knew him as “Mendeleev, the famous suitcase master”. There is a popular Russian myth that it was Mendeleev who set the 40% standard strength of vodka. However, the truth is that the 40% standard strength was introduced by Russian government in 1843, when Mendeleev was only nine years old.
#9 MENDELEEV DIDN’T GET THE NOBEL PRIZE IN CHEMISTRY DUE TO POLITICS
The Nobel Committee for Chemistry recommended Mendeleev to be awarded the Nobel Prize in Chemistry for 1906 for his discovery of the periodic system. Svante Arrhenius was a Swedish scientist who won the Nobel Prize for Chemistry in 1903 for his theory of electrolytic dissociation. He had been involved in setting up the Nobel Prizes; and he had a great deal of influence in the Academy. The Academy was supposed to approve the choice of the Committee for Chemistry but unexpectedly a member of the Nobel Committee proposed the name of French chemist Henri Moissan for the honor. Svante Arrhenius pressed for the rejection of Mendeleev and due to his influence the majority of the Academy voted for Moissan, albeit after a heated discussion. Arrhenius had a grudge against Mendeleev because Mendeleev was an outspoken critic of his dissociation theory and he made sure Mendeleev didn’t get the prize despite nominations on two more occasions.

#10 ELEMENT 101 WAS NAMED MENDELEVIUM IN HIS HONOR
Mendeleev was awarded the Davy Medal and the Copley Medal by the Royal Society of London in 1882 and 1905 respectively. He was elected a Foreign Member of the Royal Society in 1892 and in 1893 he was appointed director of Russia’s Bureau of Weights and Measures, a post which he held till his death. Dmitri Mendeleev died on 2nd February 1907 in Saint Petersburg due to influenza. He was just a few days away from turning 73. Element with atomic number 101, which was discovered in 1955, was named Mendelevium after Mendeleev. Also a crater on the Moon is named Mendeleev in his honor. Dmitri Mendeleev is considered one of the most influential chemists in history.


thanks this is half my homework done
Yo i need five this is all my homework done, well for one slide…
What you wrote made a bunch of sense. But, what about this?
suppose you were to create a awesome headline? I am not saying
your content isn’t good., but what if you added a title to possibly grab people’s attention? I mean Dmitri
Mendeleev | 10 Facts On The Father of Periodic Table | Learnodo Newtonic is
a little vanilla. You ought to glance at Yahoo’s front page
and see how they create article titles to get people to open the
links. You might add a video or a related picture or two
to grab people interested about everything’ve got to say.
Just my opinion, it could bring your posts a little
bit more interesting. – Calator prin Romania
Thanks for your suggestion. I will surely consider it.
great stuff please continue facts about these historical figures