Ernest Rutherford was a New Zealand-born British physicist who was among the leading scientists of the twentieth century. He made a paramount contribution to the sciences of Physics and Chemistry through his study of radioactivity and structure of the atom. Due to his pioneering work in the field, Rutherford is known as the Father of Nuclear Physics. Among other things, he invented a radio receiver, discovered that atoms were not indestructible, discovered alpha and beta radioactivity, interpreted the famous gold foil experiments to form the Rutherford model of the atom, discovered the atomic nucleus and the proton, and performed the first induced nuclear reaction. Rutherford was also considered the greatest experimentalist since Michael Faraday and won the 1908 Nobel Prize in Chemistry. Know more about his scientific accomplishments through his 10 major contributions.
#1 HE INVENTED AN EARLY DETECTOR OF RADIO WAVES
German physicist Heinrich Hertz proved the existence of electromagnetic waves in late 1880s. Rutherford decided to measure their effect on magnetized steel needles. His experiments led him to invent a detector for what we now know as radio waves. This radio receiver became a part of the communications revolution known as wireless telegraphy. Rutherford improved on his device and for a brief period it held the world record for the distance over which electromagnetic waves could be detected. Though Rutherford was overtaken by Guglielmo Marconi, who became the foremost figure in the field of wireless telegraphy, his receiver is still considered an important contribution.

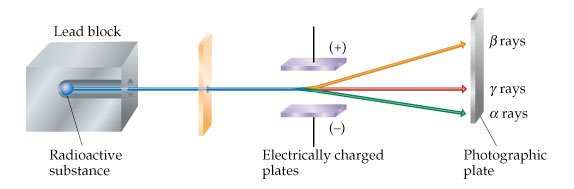
#2 He DISCOVERED ALPHA AND BETA RADIOACTIVITY
In 1898, Ernest Rutherford began studying the radiation emitted by uranium. His experiments led him to conclude that radioactivity must have at least two components; and he named them alpha and beta rays after the first two letters of the Greek alphabet. He found that alpha particles are positively charged and that they are Helium ions carrying a +2 charge. He also discovered that beta rays have more penetrating power than alpha rays. Rutherford also coined the name for gamma rays, which were discovered by French physicist Paul Ulrich Villard in 1900.
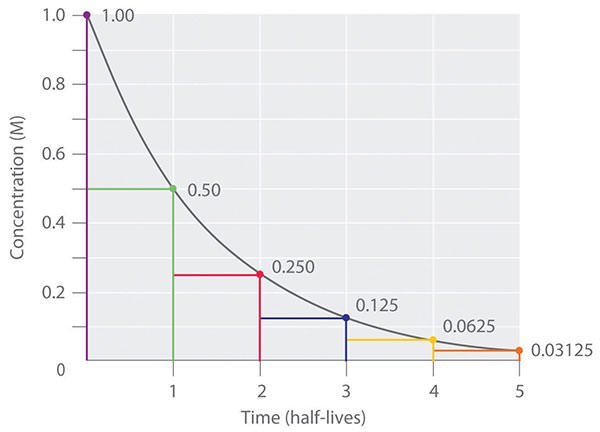
#3 HE DISCOVERED THE PRINCIPLE OF HALF-LIFE AND APPLIED IT TO RADIOMETRIC DATING
During his study of radioactivity, Rutherford found that it invariably took the same amount of time for a sample of radioactive material, radium, of any size to decay to half of its initial size. He coined the term “half-life period” for this principle, which he had discovered. Rutherford went on to apply this principle of a radioactive element’s half-life to study ‘how old things are’ by measuring the decay period of radium to lead-206, thus initiating the science of radiometric dating for age determination.
#4 He DISCOVERED THAT ATOMS WERE NOT INDESTRUCTIBLE
In 1900, Rutherford was joined by English chemist Frederick Soddy in his research on radioactivity. Together they demonstrated that it involved spontaneous disintegration of atoms into other types of atoms. They came up with the “Theory of Atomic Disintegration” to account for all their experiments. Atoms of radioactive elements breaking up, was a landmark discovery as till then it was believed that atoms were indestructible basis of all matter. It was for his investigations into the disintegration of elements and chemistry of radioactive elements that Rutherford was awarded the Nobel Prize in Chemistry in 1908.
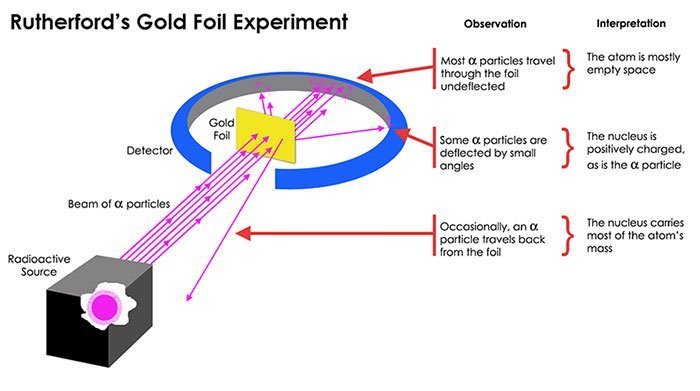
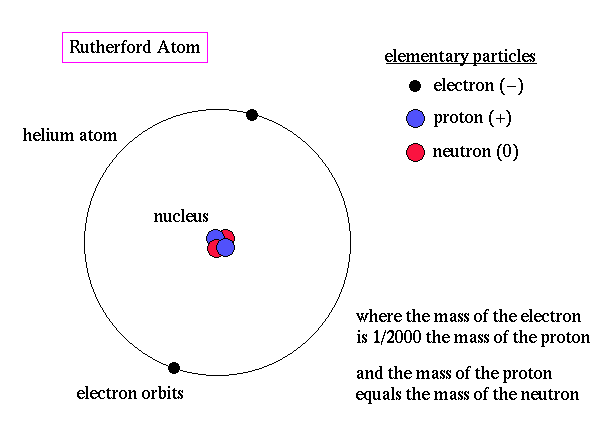
#5 HE FORMULATED THE RUTHERFORD MODEL OF THE ATOM IN 1911
Along with researchers, Hans Geiger and Ernest Marsden, Rutherford carried out one of the landmark experiments in science, known as Geiger–Marsden experiment or Rutherford gold foil experiment. Under Rutherford’s direction, Geiger and Marsden performed a series of experiments between 1908 and 1913 where they pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. They found that while most particles flew straight threw the coil, some bounced in all directions, even right back at the source. This behavior was impossible to justify by the prevalent model of the atom at the time, J. J. Thomson’s plum pudding model. Rutherford thus interpreted the data to formulate the Rutherford model of the atom in 1911.
#6 HE ANALYSED THE GOLD FOIL EXPERIMENTS TO DISCOVER RUTHERFORD SCATTERING
Geiger–Marsden experiments led Rutherford to discover and interpret the elastic scattering of charged particles that interact according to Coulomb’s inverse-square law, now known as Rutherford scattering. Rutherford scattering has since been used often to study the structure of the atom. Rutherford later also analysed inelastic scattering but it is not referred to as Rutherford scattering though he was the first to observe it. In inelastic scattering the kinetic energy of an incident particle is not conserved, unlike in elastic scattering. Rutherford’s technique also led to Rutherford backscattering spectrometry (RBS), an analytical technique used in materials science.
#7 RUTHERFORD DISCOVERED THE ATOMIC NUCLEUS
Through the gold foil experiments, Rutherford discovered that every atom contains a nucleus where its positive charge, and most of its mass, is concentrated. His model of the atom thus contained the new feature of a relatively high central charge concentrated in a small volume of the atom and responsible for most of its mass. In the model, the nucleus was orbited by low mass electrons. Rutherford’s atomic model was superseded by the Bohr atomic model in 1913, which, most prominently, applied quantum theory to it. Rutherford’s discovery of the atomic nucleus was extremely relevant and is considered his greatest contribution to science, though he received the Nobel Prize for his study of radioactivity.
#8 HE DISCOVERED THE PROTON IN 1917
In 1917, Ernest Rutherford became the first person to deliberately transform one element into another. He converted nitrogen atoms into oxygen atoms by bombarding nitrogen with alpha particles. This was the first observation of an induced nuclear reaction and is also considered the discovery of proton. The reaction was initially written as 14N + α → 17O + 1H. The result showed Rutherford that hydrogen nuclei were a part of nitrogen nuclei and he began to suspect that hydrogen nucleus may actually be a fundamental particle. In 1920, he proposed hydrogen nucleus to be a new particle and coined the term proton for it. The nuclear reaction could now be written as 14N + α → 17O + proton.

#9 HE THEORIZED THE EXISTENCE OF THE NEUTRON
Rutherford carried out calculations of the stability of atomic nuclei. In 1921, while working with Niels Bohr, he theorized that there must be a neutral particle in the nucleus to compensate for the repelling effect of the positive charges of protons by causing an attractive nuclear force. Failing such a particle, the repulsion would make the nucleus fly apart. Rutherford thus theorized the existence of the neutron and even coined the term by which it is known. His prediction was proved right with the discovery of the neutron in 1932 by English physicist James Chadwick, who had studied and worked under Rutherford.

#10 HE IS KNOWN AS THE FATHER OF NUCLEAR PHYSICS
Ernest Rutherford is known as the Father of Nuclear Physics for his work in the field including conducting the first induced nuclear reaction; establishment of the nuclear structure of the atom; and proving the nature of radioactive decay a nuclear process. His work was of paramount importance for further research and development in the field. Rutherford also provided inspiration and guidance to future scientists and an unusually large number of his students went on to win Nobel Prizes. Rutherford was also considered the greatest experimentalist since Michael Faraday.

