Sir Joseph John Thomson (1857 – 1940) is one of the most revered figures in the field of Physics. He is best known for his discovery of the electron and his experiments and work on the conduction of electricity in gases, for which he won the Nobel Prize in Physics in 1906. Thomson is revered as the founder of modern day Atomic Physics and is also known to have planted the seeds for the development of mass spectrometry. His lectures served as the precursors to Albert Einstein’s much acclaimed theory of photons. He also made extraordinary contributions to the study of matter and was a source of inspiration and knowledge for many great scientists under his tutelage, including Niels Bohr, Ernest Rutherford, Owen Willians Richardson and Charles Thomson Rees Wilson. Know more about the work of J. J. Thomson’s through his 10 greatest contributions to science.
#1 HE DID GROUNDBREAKING WORK IN CONDUCTION OF ELECTRICITY IN GASES
Cathode rays are radiation emitted when a voltage is applied between two metal plates inside a glass tube filled with low-pressure gas. In 1897, Thomson showed that cathode rays consisted of particles, electrons, that conduct electricity. This research was widely recognized as one of the most important work being done in the scientific community at the time. In 1906, Thomson was awarded the Nobel Prize in Physics “in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases.”

#2 J J THOMSON DISCOVERED THE ELECTRON
From the late 17th century onward, it was widely accepted among the scientific community that the atom was the smallest unit of matter. However, J.J. Thomson conclusively proved otherwise through his experiments with cathode ray tubes that showed that all atoms contain tiny negatively charged subatomic particles. Thomson thus discovered particles that were 1,800 times lighter than the lightest atom (hydrogen). In May, 1987 he announced the first discovery of sub-atomic particles, which Thomson would call ‘corpuscles’. Later these particles would be named electrons. Thomson’s discovery completely overturned the prevalent belief that atoms were the ‘building blocks of life’ and the smallest particles in the universe.
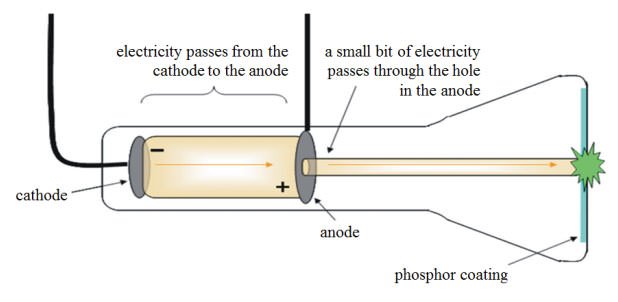
#3 HIS CATHODE RAY EXPERIMENTS AIDED THE INVENTION OF TELEVISION
The Cathode Ray formed the fulcrum of many modern day inventions such as the very first televisions. Earlier, scientists were not sure whether the electric charge from the cathode ray could be separated from the ray itself. During his cathode ray experiments, Thomson applied a magnetic field across the cathode ray tube, thus discovering that the rays were bent away by the magnetic field. This proved that the electrical charge was inseparable from the ray itself. This laid the groundwork for the development of cathode ray tubes which could be used to modulate, accelerate and deflect electron beams onto a screen to create images, thus leading to the invention of the first television sets.

#4 HE DISCOVERED THE FIRST EVIDENCE OF DIFFERENT ISOTOPES IN A STABLE ELEMENT
Isotopes are variants of a chemical element that have different numbers of neutrons. Earlier it was widely believed that stable or non-radioactive elements did not have isotopes. However, J.J Thomson proved otherwise. In 1912, Thomson and his research assist F.W. Aston conducted experiments on streams of positively charged particles. They channeled neon ions through a magnetic and an electric field on to a photographic plate. They discovered two different patches of light, which led them to conclude that neon was made up of particles with different atomic masses, or isotopes. This was the first instance of different isotopes being discovered in a stable element.
#5 HIS TREATISE ON THE MOTION OF VORTEX RINGS IS A SEMINAL TEXT ON THE SUBJECT
A vortex is a region where a fluid or gas spins around an imaginary axis that forms a closed loop. J.J Thomson’s “Treatise on the Motion of Vortex Rings” is a seminal text on the subject and it won the Adam’s Prize in 1882. The book received acclaim across the scientific community for its explanation of the subject. It is a comprehensive text book that aided the understanding of the formation, motion and interaction of vortex rings. It is still widely referred today by students and academicians alike.

#6 HE WAS THE FIRST TO EXPLAIN THOMSON SCATTERING
Thomson Scattering is the “scattering of electromagnetic radiation by a free non-relativistic charged particle. The electric and magnetic components of the incident wave accelerate the particle. As it accelerates, it, in turn, emits radiation and thus, the wave is scattered.” It is a very important concept in plasma physics and forms the basis of X-ray crystallography, inverse Compton scattering and the cosmic microwave background. This phenomenon was first explained by J J Thomson in 1903 and is named after the scientist himself.

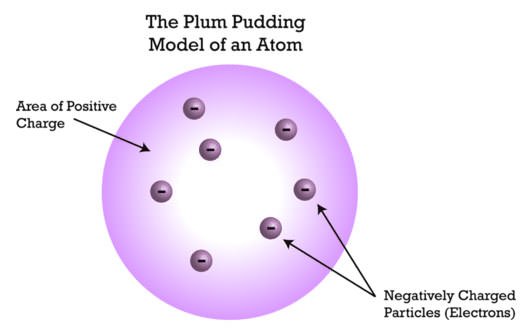
#7 HE PROPOSED PLUM PUDDING MODEL OF THE ATOM
In March, 1904, JJ Thomson proposed a model of the atom whereby the negatively charged corpuscles (electrons) were distributed in a uniform sea of positive charge and electrostatic forces determined their position. Known as the Plum Pudding Model, it played an important role in the research of atomic structure. The model got it’s name from the popular English dessert. Although the model was later disproved by Ernest Rutherford and others, it incorporated many of the ground-breaking discoveries of the time and promoted the idea of the atom as a non-inert particle which consisted of other smaller particles.
#8 HIS LECTURES FORESHADOWED EINSTEIN’S QUANTUM THEORY OF LIGHT
The wave theory of light was the most widely accepted theory of light in the 1800s. However, in 1903, Thomson proposed a discontinuous theory of light in his Silliman lectures at Yale University. This foreshadowed Einstein’s influential quantum theory of light which states that “both light and matter consists of tiny particles which have wavelike properties associated with them. Light is composed of particles called photons, and matter is composed of particles called electrons, protons, neutrons.” Einstein’s theory is very important to Quantum Physics today, but Thomson shed light on the subject even before the famous German-born physicist.

#9 HE MADE VITAL CONTRIBUTIONS TO MASS SPECTROMETRY
Sir Thomson’s efforts in measuring the mass-to-change ratio of electrons and ions was crucial to the field of mass spectrometry. By modifying an apparatus for the photographic method of recording parabolas, he managed to plot intensity against relative mass; thus creating the world’s first mass spectrometer. His plots of ion intensity against relative mass, published in 1912, were the world’s first mass spectra. In 1991, the Thomson symbol was proposed as a unit to measure mass-to-change ratio in honour of J. J. Thomson.
#10 HE VASTLY INFLUENCED THE WORK OF OTHER NOBEL PRIZE WINNING PHYSICISTS
In addition to being a very fine scientist, Sir Thomson was also a brilliant teacher and lecturer. During his time at the Cambridge University he worked closely with a number of research assistants and students who would go on to make stellar scientific discoveries of their own. This list includes Ernest Rutherford, Charles Glover Barkla, Niels Bohr, Max Born, William Henry Bragg, Francis William Aston, Owen Willans Richardson and Charles Thomson Rees Wilson. All of these scientists went on to win Nobel Prizes, six in Physics and two in Chemistry. Another major scientist that J J Thomson inspired was his own son, Sir George Paget Thomson. Thomson Junior carried forwards his father’s work and jointly won the 1937 Nobel Prize for Physics for discovering the wave-like properties of electrons.

