Born in September 1766, John Dalton was an English scientist who did pioneering work in the fields of chemistry and meteorology. He was the first to publish a paper on colour blindness and also provided great new insights into the nature of gases. He was renowned during his life though the enormous nature of his contribution was realized with further advancements in science. Here are the 10 major accomplishments of John Dalton including his remarkable contribution to chemistry and meteorology.
#1 HE MADE SEVERAL REMARKABLE METEOROLOGICAL OBSERVATIONS
Dalton’s first major achievements were in meteorology, the scientific study of atmosphere. In 1793, Meteorological Observations and Essays became his first published work. It asserted for the first time that water vapour existed independently in air and didn’t combine chemically with other atmospheric gases. It also contained his study of aurora borealis which detected the magnetic relation of the phenomenon and concluded its light to be of purely electrical origin. Dalton made important contributions to meteorology throughout his scientific career and was called the Father of Meteorology by John Frederic Daniell.

#2 He PUBLISHED THE FIRST EVER PAPER ON COLOUR BLINDNESS
John Dalton was colour blind and so was his elder brother Jonathan Dalton. In his 1794 paper “Extraordinary facts relating to the vision of colours” Dalton described the defect he had discovered in his own and his brother’s vision. This paper was the first publication on colour blindness. Though Dalton correctly recognized that the deficiency was hereditary, his theory regarding it was incorrect. Still colour blindness is sometimes referred to as Daltonism as he was the first scientist to thoroughly investigate the defect.
#3 JOHN DALTON DID PIONEERING WORK IN HYDROLOGY
Dalton’s 1799 paper proposed after research and estimated calculations that the quantity of rain and dew are equal to the quantity of water carried off by evaporation and by the rivers. It also contained the earliest definition of the ‘dew-point’ and settled for all practically purposes the controversy over the origin of springs by his conclusion that they are fed by rain. This paper was an important step in the development of quantitative hydrological cycles. Due to John Dalton’s contribution, the Dalton Medal is given to hydrologists by the European Geophysical Society for distinguished research in the field.

#4 HE PROVIDED GREAT NEW INSIGHTS INTO THE NATURE OF GASES
In 1802, John Dalton’s ground-breaking research, which provided great new insights on the nature of gases, was published. In it he noted correctly that all gases could be liquefied provided their temperature was sufficiently low and pressure sufficiently high; and that all gases expand the same quantity by heat. He also came up with what is known as Dalton’s law of evaporation. It states that the rate of evaporation is proportional to the difference between the saturation vapour pressure at water temperature and the actual vapour pressure in air.
#5 HE OBSERVED WHAT IS KNOWN AS DALTON’S LAW OF PARTIAL PRESSURES
In 1801, John Dalton found that volume of all gases he studied increased proportionally with rise in temperature when pressure was held constant (VαT at constant P). The law however bears the name of French scientist Jacques Charles, who had formulated it earlier but never published the results. In 1803, Dalton published his Law of Partial Pressures, which states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. Also known as Dalton’s Law, it is commonly applied in looking at the pressure of a closed container of gas and water.
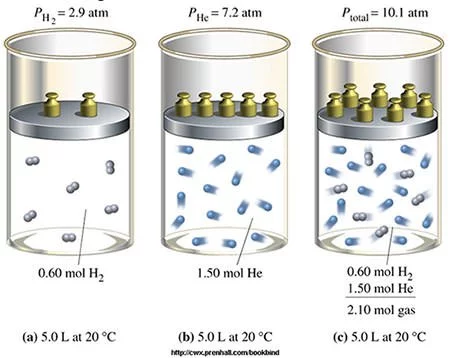
#6 HIS LAW OF MULTIPLE PROPORTIONS IS ONE OF THE BASIC LAWS OF STOICHIOMETRY
Two important laws dealing with chemical reactions emerged near the end of the 18th century – Antoine Lavoisier’s law of conservation of mass and Joseph Proust’s law of definite proportions. Through the study of these laws and experimentation John Dalton developed his law of multiple proportions, which states that if two elements can be combined to form a number of possible compounds, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers. The three laws mentioned above form the basis of Stoichiometry, i.e. the calculation of relative quantities of reactants and products in chemical reactions.
#7 HE PROPOSED THE FIRST TRULY SCIENTIFIC ATOMIC THEORY
Dalton’s law of multiple proportions, which he announced in 1803, became the basis for his famous Atomic Theory which he proposed later that year. The 5 main points of Dalton’s atomic theory are: elements are made of extremely small particles called atoms; atoms of a particular element are identical; atoms cannot be created, destroyed or split; atoms of different elements combine in simple whole-number ratios to form chemical compounds; and in a chemical reaction, atoms link to one another, or separate from one another. Dalton’s theory was the first truly scientific theory of the atom reached through analysis and experimentation.
#8 HiS ATOMIC THEORY LAID THE FOUNDATION OF MODERN CHEMISTRY
Though later research found that atoms of the same element are not necessarily identical as they can have different masses (isotopes) and that atoms can be split in nuclear reactions; Dalton’s atomic theory holds good in several aspects even today and it remains valid for chemical reactions. Also Dalton’s theory laid the foundation of modern chemistry and the basis on which future scientists made numerous other highly significant discoveries.
#9 DALTON WAS THE FIRST TO CALCULATE RELATIVE ATOMIC WEIGHTS
On the basis of his atomic theory, John Dalton calculated the first relative weights of atoms. He estimated the atomic weights according to the mass ratios in which they combined; with the hydrogen atom taken as unity. He proceeded to print the first published table of relative atomic weights. Published in 1803, his first list contained only 6 elements. This was followed by a 20 elements list in 1808 and a 36 element list in 1827. In the long run atomic weights would provide the key means of organizing elements into the periodic table.

#10 HE RECEIVED SEVERAL HONOURS INCLUDING THE ROYAL MEDAL
John Dalton served as president of the Manchester Literary and Philosophical society (the “Lit & Phil”) from 1817 till his death. In 1822, he was made a fellow of the Royal Society of London and in 1826 he was awarded the Society’s Royal Medal for his Atomic Theory. In 1830, Dalton was elected one of only eight foreign members of the French Academy of Sciences and in 1834 he was elected a Foreign Honorary Member of the American Academy of Arts and Sciences. Also Francis Leggatt Chantrey’s statue of him made John Dalton probably the only scientist who got a statue in his lifetime.


He was a great man
His research were wonderful and as the matter of fact the basis in chemistry
Super Dalton
wonderful work done by Dalton,,